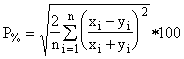 (1)
(1)Methodology for Chemical Speciation Measurements in the IMPROVE Network
Author: Robert Eldred, CNL, University of California, Davis (raeldred@ucdavis.edu)
Date: February 1998
ABSTRACT
The IMPROVE (Interagency Monitoring of Protected Visual Environments) network collects PM2.5 particles on Teflon, nylon, and quartz filters using a modular, cyclone-based sampler with critical orifice flow control. Understanding the protocols of IMPROVE is important because IMPROVE will continue to provide background monitoring at Class I areas, and because the protocols for measuring chemical species in the Federal Reference Method network at urban and suburban sites will probably be similar. The IMPROVE filters are analyzed by seven methods to obtain concentrations of mass, elements H and Na-Pb, ions NO3- and SO4=, organic and elemental carbon, and the coefficient of absorption. The replicate precision for most analytical methods is approximately 4%. The artifacts for all methods except carbon are small or zero. The artifact problem for organic carbon is minimized by allowing each filter to reach saturation prior to collection. Redundant measurements by the three filters permit improved quality control. Comparisons of sulfur with sulfate and organic carbon with organic hydrogen permit assurance that (1) the calculated uncertainties are reasonable, (2) the analytical methods are correctly calibrated, (3) the flow rates of the various modules are in agreement, and (4) the individual concentrations are valid.
INTRODUCTION
The major components of PM2.5 are sulfate, nitrate, organics, elemental carbon, and soil. For coastal sites, NaCl constitutes a sixth major category. A full chemical speciation may be defined as measuring these five (or six) components plus trace elements. The first national monitoring network with full chemical speciation was IMPROVE (Interagency Monitoring of Protected Visual Environments), which began in 1987.1 The purpose of the program was to enable the Environmental Protection Agency (EPA) and the Federal Land Managers to protect Class I visibility areas from increased visibility impairment, as required by the Clean Air Act of 1977. Participants in the program include the National Park Service, Forest Service, and Fish and Wildlife Service, Bureau of Land Management, Department of Energy, Tahoe Regional Planning Agency, interstate agencies, and several states. The methodology is to collect samples on Teflon, nylon, and quartz filters, which are analyzed by several methods including X-ray Fluorescence (XRF), ion chromatography (IC), and Thermal/Optical Reflection (TOR) carbon analysis. The IMPROVE network currently includes 63 sites with full speciation and 12 sites with partial speciation.
In 1993, EPA began a full speciation visibility monitoring network at about 10 sites in small urban communities in the eastern United States, the Clean Air Status and Trends Network (CASTNet). The collection and analysis for PM2.5 followed the model of IMPROVE. Both CASTNet and IMPROVE use cyclones to define the 2.5µm particle size cutoff.
EPA will begin the PM2.5 Federal Reference Method (FRM) network in 1998 to determine compliance toward a health-based mass standard. Eventually, this network will encompass 1100-1500 urban sites. Associated with this will be a chemical speciation network at about 300 of the sites to provide information on the composition of the PM2.5 mass. This network will collect samples on Teflon, nylon, and quartz filters and use analytical methods similar to those of IMPROVE.
In 1998, in response to the proposed Regional Haze regulations, EPA will expand the IMPROVE network in include nearly all of the 156 mandatory Class I areas. Addition sites in remote areas will be operated by states and FLM’s as part of the Regional Haze regulations. These may be operated as part of the IMPROVE network, or operated independently.
Thus, in the next two years, the number of national monitoring sites with full chemical speciation will increase from about 75 to nearly 500. All sites will collect PM2.5 particles on equivalent samplers. The networks should follow similar analytical methods and quality assurance procedures. This will ensure compatible data between networks and permit a unified national database.
SAMPLE COLLECTION
The current sampling sites in the IMPROVE network are indicated in Figure 1. The sites are separated into three groups: remote sites with full speciation, urban sites with full speciation, and remote sites with partial speciation. The full speciation sites sample PM2.5 particles on Teflon, nylon, and quartz filters. The measured variables for the three filter are shown in Table 1. PM10 samples are included at most of these sites to monitor the contribution of coarse particles to extinction. The partial speciation sites have only a Teflon filter or Teflon plus nylon filters. Flow audits are performed quarterly by the site operators or during the annual maintenance.
The IMPROVE sampler uses a cyclone operating at 22.8 LPM to define the PM2.5 size cut.2 The flow rate is controlled using a critical orifice. The flow rate is measured by two gauges at the beginning and end of the sample period. One gauge measures the flow rate ahead of the filter while the other measures it behind, permitting a check that the cassettes are properly installed. Based on third-party audits, the uncertainty in flow rate and volume is 3%. This value is used for all samples.
Two 24h samples are collected each week. In 1998, this will change to the national 1 day-in-3 schedule. Sample changes are performed weekly by on-site operators provided by the federal land managers. All filters are kept in cassettes and changed in the central laboratory. The recovery rate for the network averages 95%. Most of the losses are associated with the inability of the operator to change the sample during the appropriate 48h window.
The quality assurance procedures for sample collection include comparing the two measurements of the flow rate ahead of and behind the filter. Flow rates are compared to historical values to monitor any changes. The data validation procedures include additional checks on the flow rate and correct sample collection parameters.
SAMPLE ANALYSIS
X-ray Analyses—Teflon Filters
The IMPROVE Teflon filters are analyzed by a combination of X-ray Fluorescence (XRF) and Particle Induced X-ray Emission (PIXE). Both systems are nondestructive, permitting later re-analysis. The Mo-anode XRF system is used for elements Fe and heavier, while the PIXE system is used for the elements Na to Mn. The PIXE analyses are equivalent to XRF analyses using one or more anodes of lower energy. In IMPROVE, the XRF and PIXE analyses are performed in large batches every quarter, in order to optimize quality control.
XRF and PIXE analyses give spectra in which the characteristic x-ray peaks are superimposed on background x-rays.3 This background can be simulated by analyzing a clean filter. The peaks corresponding to a given element are at a fixed energy. The concentrations for a given element can be calculated from the number of x-rays in the peak. The uncertainty in the concentration is the quadratic sum of the statistical uncertainty for that specific peak and the calibration uncertainty for the system.
The XRF and PIXE systems are calibrated by a series of elemental standards that are NIST-traceable. The complete calibration is repeated whenever the physical configuration is changed. A partial recalibration is performed at the beginning of each quarterly session. This is followed by the reanalysis of a set of 30 filters from the previous quarterly session. Correlation plots are examined for selected elements. The relative precision is defined by Equation 1, where x and y are the original and replicate concentrations.
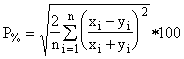 (1)
(1)
The reanalysis is considered acceptable only if the precision for key elements, such as S for PIXE and Fe for XRF, is approximately 4%. (For these elements, the statistical uncertainty is negligible, so the calculated precision represents the calibration uncertainty for the system.) The regular analyses are performed only after proper agreement for calibration standards and reanalysis filters. The partial calibration and reanalysis is repeated in the middle and at the end of the session.
No significant concentration for any element is observed on field blanks, so no elemental artifact is subtracted.
The concentrations are corrected for the loss of x-rays as they pass through the sample. The corrections the same for PIXE and XRF. The main correction is associated with the particle containing the element. The correction factor involves the energy of the x-ray, and the average size and composition of the particles for that element. Because this involves assumptions about the particle size and composition, the correction is not well defined. Fortunately, the corrections are generally small. The largest correction for PM2.5 elements is for aluminum (17%), silicon (9%), and sodium (7%). The factor drops to 2% for calcium and about 1% for iron and sulfur. There is an additional correction for layering of particles. Except for a few heavily loaded samples, the factor is less than 5%. The effect of the uncertainty in either correction on the uncertainty in the concentration is well below the analytical precision of 4%.
The analytical uncertainty for an elemental concentration is the quadratic sum of the volume uncertainty of 3%, the calibration uncertainty of 4%, and the statistical uncertainty from the counts in the spectrum. The median relative uncertainty for all major elements is between 5% and 10%. The median relative uncertainties for trace elements Zn, Br, Pb, Cu, and Se range from 7% to 17%.
When the concentration a given element is below a minimum detectable limit (mdl) , the x-rays cannot be observed above the background. This ranges from about 0.03 ng/m3 for elements near Se to 13 ng/m3 for Na. Sulfur is above the mdl on every sample. All major elements are found on at least 90% of the samples. The trace elements Zn, Br, and Pb are found on 90%-100% of the samples, while Se is found on about 80%. The mdl for each measurement is retained in the database.
The XRF and PIXE concentrations of key common elements are compared for every sample. Figure 2 indicates the comparison for iron for samples collected during summer 1996. The slope minimizing perpendicular deviations is 1.008 ± 0.003, with an intercept of 0.003 ± 0.003, and the correlation (r2) is 0.996. If multiple XRF analyses are performed, similar comparisons for overlapping elements should be made.
Hydrogen Analyses—Teflon Filters
The concentration of hydrogen is determined by Proton Elastic Scattering Analysis (PESA), run concurrently with the PIXE analysis. Teflon is well suited for the analysis as there is no hydrogen. The system is calibrated using a mylar standard. Re-analyses are performed in the same manner as PIXE and XRF. The calibration uncertainty is similar to than for PIXE and XRF, 4%. The analytical uncertainty is determine from the counts in the spectrum. The median relative uncertainty for H is 6%. H is above the minimum detectable limit on 99% of all samples.
The main use of hydrogen is to obtain a measurement of organic as a quality assurance check of organic estimated from organic carbon from the quartz filter, as discussed in the section on data validation. The possible sources of hydrogen on the filter are organics, sulfate, and nitrate. Since the analysis is conducted in vacuum, all water is removed prior to analysis. If the nitrate on the Teflon filter is small compared to sulfate and if the sulfate is assumed to be present as (NH4)2SO4, the organic hydrogen is given by Equation 2.
![]() (2)
(2)
If NH4+ is measured on the corresponding nylon filter, the equation can be corrected for the presence of H2SO4 and (NH4)HSO4.
Ion Chromatography Analyses—Nylon Filters
The concentrations of SO4=, NO3-, and Cl- are determined from the nylon filters using ion chromatography at Research Triangle Institute. Since the nylon filter follows a carbonate denuder, the NO3- is particulate nitrate. An analytical uncertainty of 5% is determined from replicate analyses of the solution. Artifacts are determined from field blanks. The artifacts for sulfate and nitrate correspond to about 17 ng/m3, and for chloride to 6 ng/m3. The sulfate and nitrate artifacts are small compared to the median concentrations of 900 and 170 ng/m3, respectively. The chloride artifact is small to the concentrations obtained at marine sites, but is about one-half of the measured chloride on the filter elsewhere. The uncertainty in the artifact is set equal to the standard deviation of the field blanks. The relative uncertainty is smaller than 50% on nearly all of the samples for sulfate and nitrate, but less than one half of the samples for chloride. The total uncertainty in the concentration is a quadratic sum of the collection, analytical, and artifact uncertainties. The median relative uncertainties are 5% for nitrate and sulfate and 66% for chloride.
Carbon Analyses—Quartz Filters
The concentration of carbon is determined from the quartz filters using the Thermal/Optical Reflectance Method at Desert Research Institute.4 For the IMPROVE samples, the carbon is separated into 4 organic fractions and 3 elemental fractions on the basis of temperature and atmosphere, as listed in Table 2. A 0.5 cm2 punch is taken from the quartz filter and placed in the analyzer. All oxygen in the oven atmosphere is removed with helium. The oven temperature is raised in 4 steps to 550°C in a 100% He atmosphere. The carbon evolved in each step is converted to methane, which is quantified by a flame ionization detector. All carbon that evolves without added oxygen is defined to be organic. Two percent oxygen is added to the atmosphere with the temperature remaining at 550°C. Two additional temperature steps of 700°C and 800°C are made. All carbon that evolves after oxygen is added is defined to be elemental. The optical absorption of the sample changes during the analysis, increasing during the steps without added oxygen and decreasing after oxygen is added. (On some samples, the absorption begins decreasing during the last organic step, O4.) The optical absorption of the samples is monitored throughout the analysis by the reflectance of a laser beam. The darkening is assumed to be produced by the pyrolysis of organic carbon into elemental carbon, which remains on the filter. The elemental carbon evolved before the reflectance returns to the initial value is assumed to actually be organic and is placed in a separate category, OP. In about 90% of the cases, the initial reflectance is obtained during the first EC step, with the remaining 10% occurring during the second EC step. Generally, it is convenient to combine these eight fractions into two variables, organic carbon and elemental carbon, given by Equations 3 and 4.
OC = O1 + O2 + O3 + O4 + OP (3)
EC = E1 + E2 + E3 - OP (4)
The separation between OC and EC is based on the need for oxygen and on the optical reflectance. EC will include elemental carbon, but may include other carbon species, such as heavy organics and carbonate.
The analytical precision is determined by analyzing a second punch from the filter. Figure 3 shows good replicate precision for organic and elemental carbon, for all samples collected between March 1996 and February 1997. The analytical precision, after omitting the very lightly loaded samples, is 4% for organic carbon and 7% for elemental carbon.
There is an artifact on the quartz filter associated with the adsorption of organic gases from the atmosphere.5, 6 Tests at Davis and in the field suggest that the artifact reaches a saturation limit of about 2.9 µg/cm2. This suggests that there is a finite number of sites on the filter that attract the organic gases. This saturation will occur by passive contact with the atmosphere as well as by drawing air through. Because the filters are in cassettes for several weeks before sampling, the quartz filters in IMPROVE reach saturation before sampling. Therefore, no additional artifact is acquired during sampling. Thus, the secondary filters (which follow the primary quartz filters) have similar organic concentrations to those on field blanks, which have no air passing through. This can be seen in Figure 4. Measurements made throughout the network show no significant difference in artifact between sites. Because the artifact does not depend on the amount of organic gas in the airstream, it is not necessary nor desirable to subtract the artifact from the corresponding secondary filter. Combining all secondary filters produces a more precise artifact value. Currently over 100 secondary filters are analyzed each quarter and used to calculate a seasonal-median artifact.
The test of whether the calculate artifact is correct is the behavior of the resulting concentrations in the limit of low concentrations. In general, with a wide variety of sites, it is reasonable that the low end of the distribution should occur at zero concentration. This is the case for the IMPROVE data for OC as well as for most major elements and ions. Figure 5 shows the lower end of the distributions for OC and EC, after subtraction of the artifact, for all samples collected in 1996. In both bases, the distribution approaches zero. Less than 1% of the samples in either case have a zero or negative concentration.
The major source of uncertainty for organic concentrations is associated with the variation in the artifact. Figure 6 shows the distribution of organic carbon on the secondary filters. The variation represents the variation in the saturation limit, not on the TOR analysis. This variation is larger than desired, but acceptable. For the median sample, the uncertainties in the artifact is about 3 times the analytical uncertainties of 4% and 7%. The only way to decrease this artifact uncertainty will be to treat the clean filters with a gas that saturates the adsorption sites. The mass variation would probably be decreased by using a single organic compound. Better yet would be to use an inorganic gas. The median calculated uncertainty is 15% for organic carbon and 18% for elemental carbon. The uncertainty exceeds 50% for about 10% of the samples for both OC and EC.
Data Validation and Quality Assurance by Redundant Measurements
Comparable variables on different filters provide quality control in the data validation procedures beyond the normal procedures for sample collection and analysis. The most important comparison is between the sulfur measured on the Teflon filter and the sulfate measured on the nylon filter. Figure 7 shows the comparison for all sites for one quarter, summer 1996, for all sites in the network. The correlation (r2) is 0.99 and the slope is 1.00 ± 0.003, with a very small intercept, 0.05 ± 0.02 µg/m3. This comparison can be used in several ways in the data processing.
1. Comparing the measured differences to the calculated uncertainties checks the validity of the calculations. An appropriate measure is the c2 statistic, given by Equation 5. This examines the ratio of the differences to the calculated uncertainties of both variables. A c2 value near unity indicates that the calculated uncertainties properly represent the observed differences.. For this comparison, c2 is 1.05, indicating a good agreement.
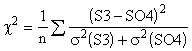 (5)
(5)
2. The comparison for all sites for the quarter verifies that both analytical systems are calibrated properly. An incorrect calibration would result in a slope significantly different from unity.
3. The comparison for a single site for the quarter verifies that the two modules have a consistent flow rate calibration. Again, if one calibration were incorrect, the slope for that site would be different from unity. Note that it is possible for both modules to have an incorrect calibration, caused by an incorrectly calibrated audit device.
4. The fact that the overall comparison is good, permits a comparison for each sample. This is the most important use of the sulfate comparison in data validation. In the IMPROVE protocol, the collection parameters and all aspects of handling and analysis are examined each outlying point. In most cases, the problem can be identified and corrected, so that the correct concentrations can be retained in the database. Occasionally, the measurements on one or all of the filters must be invalidated.
A comparison between the Teflon and quartz filters is possible for many sites because of the relationship between organic carbon and organic hydrogen. The organic hydrogen is estimated from the total hydrogen measured on the Teflon filter, after removal of other sources of hydrogen. The simplest calculation is to assume that the only inorganic hydrogen in a vacuum is from (NH4)2SO4. Figure 8 compares the two estimates of organic mass for 23 sites where sulfate is dominantly ammonium sulfate, and where nitrate and marine sulfur are small. Factors of 1.4 and 13.75 are used convert from OC and organic hydrogen to total organic. The x-variable is organic from the quartz filter, while the y-variable is the Teflon filter. The correlation (r2) is 0.96 and the slope is 1.00. When the organic concentration is low, the uncertainties in both measurements tend to be relatively large. Nevertheless, this comparison is often helpful in identifying outliers. Note that when discrepancies occur for the sulfate comparison, the organic comparison can be useful in determining whether the Teflon or nylon values were in error. The organic comparison can be made in sites with acidic sulfate if ammonium ion is also measured. The comparison must be made with caution when the nitrate concentrations are high.
Because all the major components are measured, it is possible to determine a reconstructed mass, which can be compared to the gravimetric mass. Nitrate presents a problem, since a major fraction of the particulate nitrate may be lost from Teflon filters during sample collection. For comparison with gravimetric mass it is preferred to exclude the nitrate from the nylon filter. The reconstructed mass is the sum of sulfate and soil from the Teflon filter, and organic and elemental carbon from the quartz filter. The estimates of these components is discussed in a companion paper in this volume.7 The sulfate is assumed to be (NH4)2SO4, which is 4.125 S. Soil (crustal material) is the sum of the soil-derived elements with their normal oxides. Organic is assumed to be 1.4 times OC. No factor is used for elemental carbon. The left side of Figure 9 shows the comparison for all sites in the network for summer 1996. There are 1351 points. The correlation (r2) is 0.97. The slope is 0.86, indicating that 14% of the mass is not accounted for. A small portion of this is the residual nitrate on the Teflon filter. The majority of the missing mass is presumed to be water on the Teflon filter at the time of gravimetric analysis. However, the missing mass would be less if the multiplicative factor for organic carbon were larger than 1.4.7
This plot provides information about the gravimetric mass artifact. If the correct artifact is used, then the intercept will approach zero. Rather than use the intercept for a regression based on all samples, it is better to examine the lower end of the plot. The right side of Figure 9 indicates that the mass concentration approaches zero when the reconstructed mass approaches zero.
CONCLUSIONS
The analytical precisions of the x-ray, ion chromatography, and thermal/optical reflectance carbon methods all are approximately 4%, based on replicate measurements. These precisions should be adequate for any future national networks.
There are no measurable artifacts on the Teflon filter. The sulfate and nitrate artifacts on the nylon filter are small compared to typical ambient concentrations. The artifacts on the quartz filter for both organic and elemental carbon are large relative to the ambient concentrations. Because of the delay time between loading the filters and sampling that is inherent in a typical monitoring network, the organic carbon artifact reaches a saturation value prior to sampling. The uncertainty in the artifact is larger than in the other analytical methods, but is still acceptable. In situations with low ambient concentrations of organic or elemental carbon, the relative uncertainty in the measurement may be large.
The measurement of similar variables on different filters provides extremely valuable quality assurance checks. The most important comparison is between sulfur measured on the Teflon filter with sulfate measured on the nylon filter. A second comparison is possible in the IMPROVE network because of the measurement of elemental hydrogen on the Teflon filter; this can be used to calculate organic mass in many environments that can be compared to the organic carbon from the quartz filter. These comparisons permits several checks on the accuracy and precision of the various systems. They also play a key role in the validation procedures for individual sample by identifying outlier pairs. In most cases, the problem is correctable, so that the concentrations can be retained in the database.
Finally, chemical speciation measurements can provide a valuable check of the gravimetric mass concentration for every sample. This will be especially important in the FRM network, since PM2.5 mass measurements are used to measure compliance.
REFERENCES
1. Joseph, D.B.; Metsa, J.; Malm, W.C.; Pitchford, M.L. "Plans for IMPROVE: A federal program to monitor visibility in class I areas," In Visibility Protection: Research and Policy Aspects, P.S. Bhardwaja, Editor, APCA, Pittsburgh, PA, 1990, pp. 113-125.
2. Eldred, R.A.; Cahill, T.A.; Wilkinson, L.K.; Feeney, P.J.; Chow, J.C.; Malm, W.C.,. "Measurement of fine particles and their chemical components in the IMPROVE/NPS networks," In Visibility and Fine Particles, C.V. Mathai, Editor, A&WMA, Pittsburgh, PA, 1990, pp. 187-196.
3. Harrison, J.F.; Eldred, R.A. “Automatic data acquisition and reduction for elemental analysis of aerosol samples,” Advances in X-Ray Analysis 1974, 17, 560‑583.
4. Chow, J.C.; Watson, J.G.; Prichett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. “The DRI thermal/optical reflectance carbon analysis system: description, evaluation, and applications in U.S. air quality studies,” Atmos. Environ. 1993, 27, 1185-1201.
5. Turpin, B.J.; Huntzicker, J.J.; Hering, S.V. "Investigation of organic aerosol sampling artifacts in the Los Angeles basin," Atmos. Environ. 1994, 28, 3061-3071.
6. McDow, S.R.; Huntzicker, J.J. "Vapor adsorption artifact in the sampling of organic aerosol: face velocity effects," Atmos. Environ. 1990, 24A, 2563-2571.
7. Eldred, R.A., Feeney, P.J.; Wakabayashi, P.K. “The Major Components of PM2.5 at Remote Sites across the United States,” this volume.
Table 1. Filters and analytical methods using in the IMPROVE protocol. The nylon filter follows a carbonate denuder to remove HNO3, so only particulate nitrate is collected.
|
filter |
analysis |
measured variables |
|
Teflon |
gravimetric |
PM2.5 mass |
|
|
XRF and PIXE |
major elements (S, Al, Si,
K, Ca, Ti, Fe, Na, Cl) |
|
|
proton elastic scattering |
elemental H |
|
|
integrating plate/sphere |
coefficient of absorption (for visibility, not health) |
|
nylon |
Ion Chromatography |
NO3-, SO4=, Cl-, NH4+ |
|
quartz |
thermal / optical reflection |
organic and elemental carbon (8 fractions) |
Table 2. Temperature fractions in the Thermal/Optical Reflectance Analysis. The fractions E1 and E2 include the pyrolized component OP.
|
Fraction |
temperature range |
atmosphere |
|
O1 |
to 120°C |
100% He |
|
O2 |
120 - 250°C |
100% He |
|
O3 |
250 - 450°C |
100% He |
|
O4 |
450 - 550°C |
100% He |
|
E1 |
550°C |
2% O2 |
|
E2 |
550 - 700°C |
2% O2 |
|
E3 |
700 - 800°C |
2% O2 |
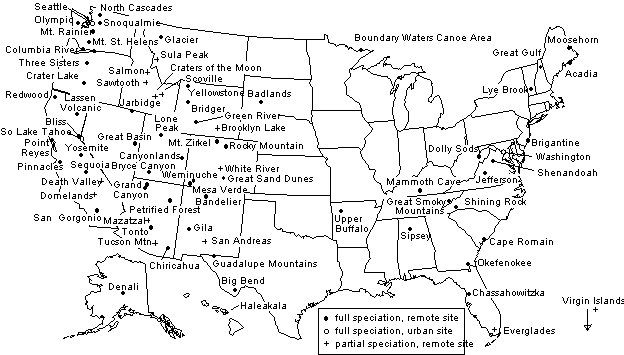
Figure 1. IMPROVE sampling sites in operation January 1998.
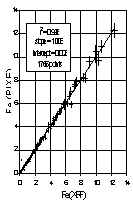
Figure 2. Comparison of Fe by XRF and Fe by PIXE for all samples collected during summer 1996. The solid line is the best-fitting line minimizing perpendicular deviations. The error bars indicate the calculated uncertainty for each measurement.
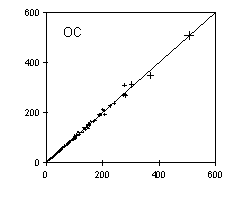
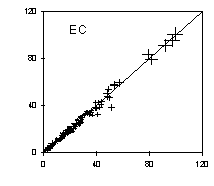
Figure 3. Replicate measurements of organic and elemental carbon by TOR. The units are µg per filter. The 1:1 line is shown. The error bars indicate a 4% uncertainty for OC and 7% for EC.
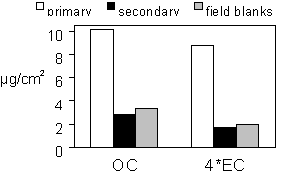
Figure 4. Median concentrations of primary, secondary, and field blank filters. From all samples collected in 1996.
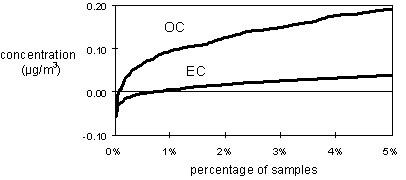
Figure 5. Lower end of distributions of organic and elemental carbon concentrations after subtraction of artifacts, showing that the chosen artifacts are reasonable. Based on all data from IMPROVE network collected in 1996.
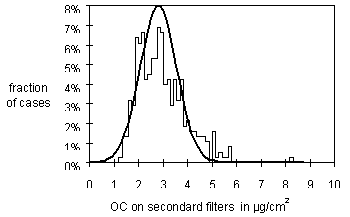
Figure 6. Distribution of organic carbon artifact concentrations from secondary filters. From all samples collected in 1996. The curve is a normal distribution about the median of the concentrations.

Figure 7. Comparison of sulfur from the Teflon filter, measured by PIXE, with the sulfate from the nylon filter, measured by IC, for all samples collected during summer 1996. The solid line is the best-fitting line minimizing perpendicular deviations.
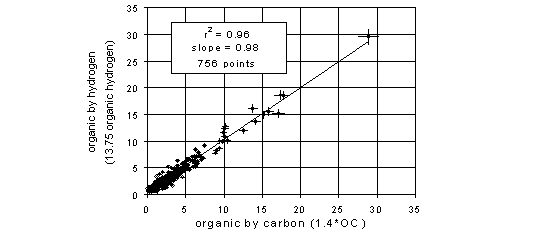
Figure 8. Comparison of organic from the Teflon filter, estimated from H and S, with the organic from the quartz filter, estimated from OC, for all samples collected at 30 western sites (excluding 6 sites with elevated nitrate) during summer 1996. The bars indicate the calculated uncertainties for each measurement. The solid line is the best-fitting line minimizing perpendicular deviations. The dotted line indicates a 1:1 ratio.

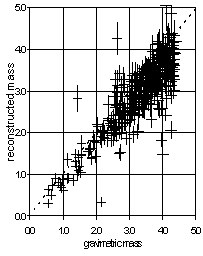
Figure 9. Comparison of reconstructed and gravimetric mass for all samples collected during summer 1996. The reconstructed mass is the sum of sulfate and soil from the Teflon filter, and organic and light carbon from the quartz filter. The bars indicate the calculated uncertainties for each variable. The solid line is the best-fitting line minimizing the y deviations. The dotted lines indicate a 1:1 ratio. The left side shows all samples, while the right side shows the lower 25% of the samples.